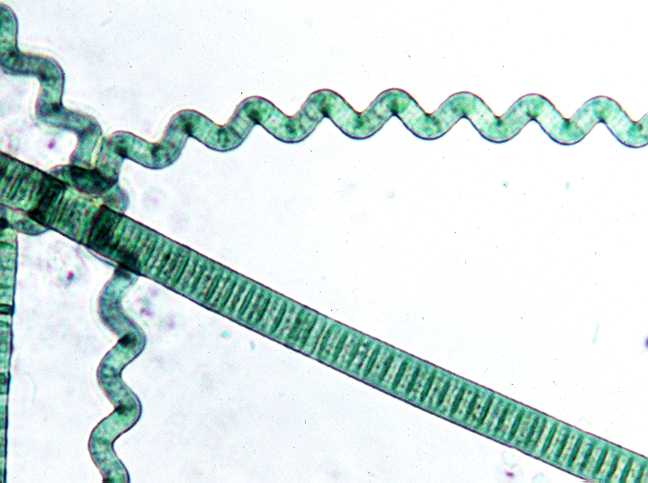
For my research project this semester, I wanted to do something regarding algae with a focus on genetics. So, I came up with a toxicity bioassay based on J.I. Nirmal Kumar's study, "Toxicity analysis of pesticides on cyanobacterial species by 16s rDNA molecular characterization."
Once I isolate single colonies of my algal species,
Nannochloropsis sp. and
Arthrospira platensis, I will do a PCR (polymerase chain reaction) of the 16s RNA gene. Then I will subject the cultures to increasing amount of foliar pesticide, tebuconazole, similar to Kumar's study. However, I will be using a home use formula of the same brand, Kumar used a commercial agriculture formulation. The idea is to continue doing a PCR and record any mutations in the RNA structure; such as insertions, deletions or mismatching of base pairs.
Here are a few photos of the starter cultures.
citation: Kumar, J.I. Nirmal, et al. "Toxicity analysis of pesticides on cyanobacterial species by 16s rDNA molecular characterization."
Proceedings of the International Academy of Ecology and Environmental Sciences 3.2 (2013)





.jpeg)

.jpeg)